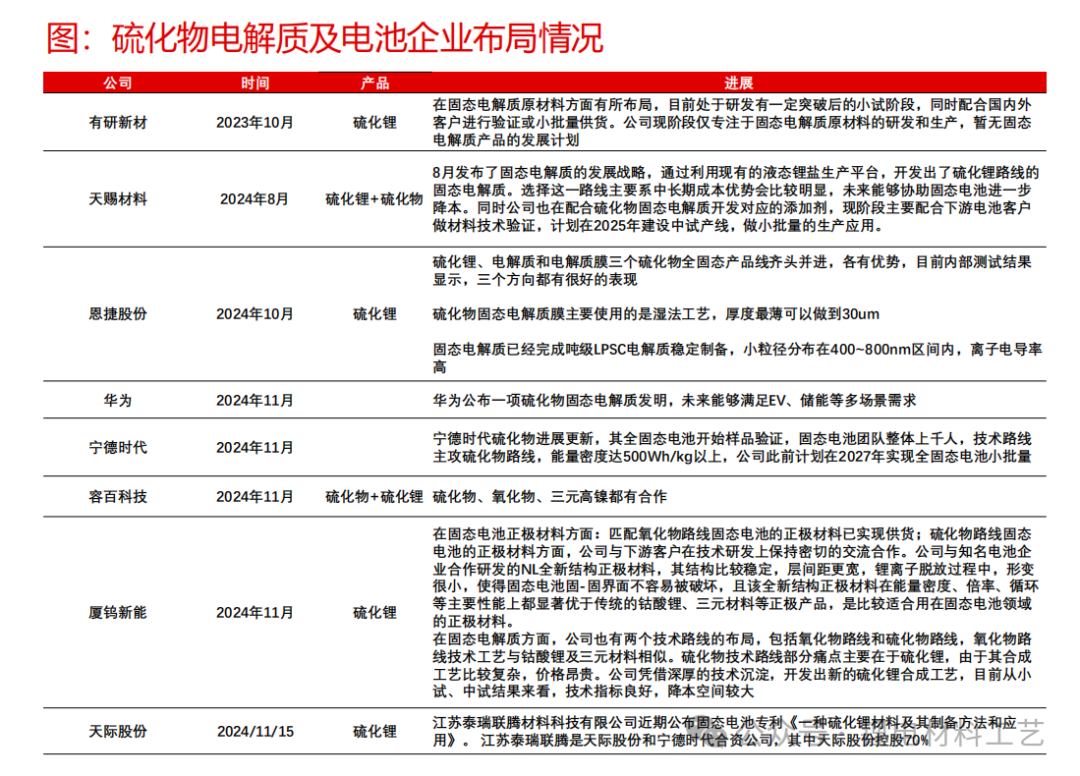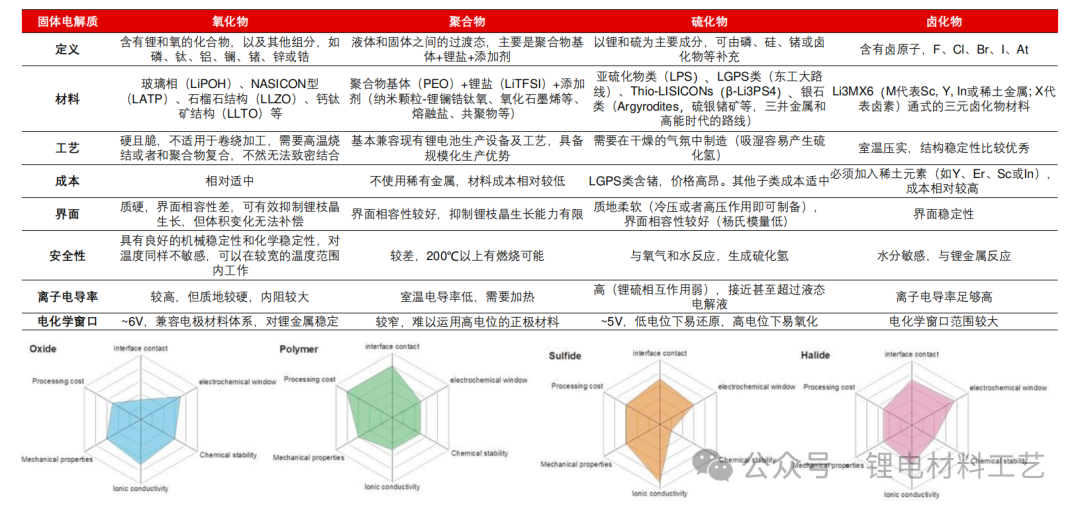
➢ Sulfide electrolyte has ideal ionic conductivity (compared with liquid electrolytes), good electrode material compatibility (a wide electrochemical window), and is one of the most ideal solid electrolytes at present, with the greatest development potential in all solid-state batteries. However, there are many problems that need to be solved urgently:
➢ 1) The interface is unstable, and side reactions are prone to occur, causing greater impedance;
➢ 2) Chemical reactions are very likely to occur in alkaline and aqueous environments to produce hydrogen sulfide;
➢ 3) The addition of rare earth metals has greatly increased the processing cost and material cost.
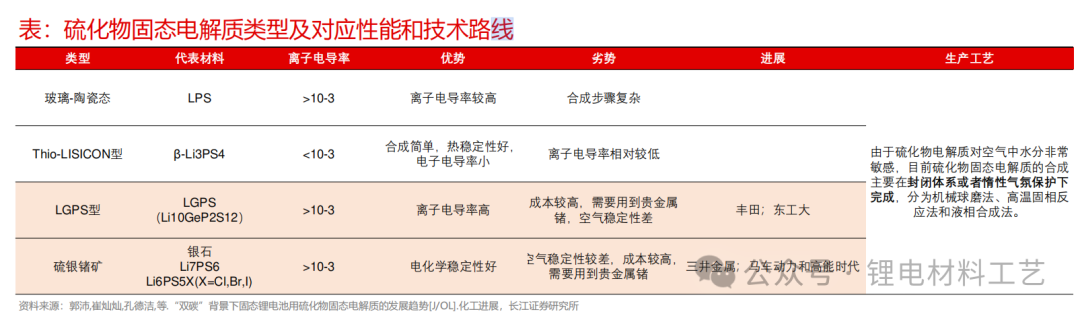
➢ LGPS type and sulfur silver germanium ore type have high ionic conductivity, while sulfur silver germanium ore type SE has low cost and high stability characteristics, and has good development prospects.
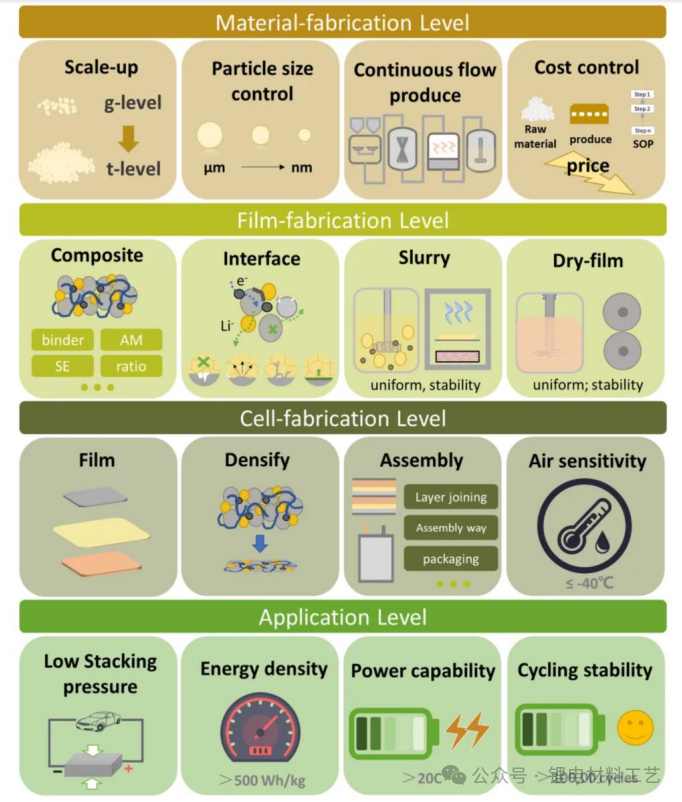
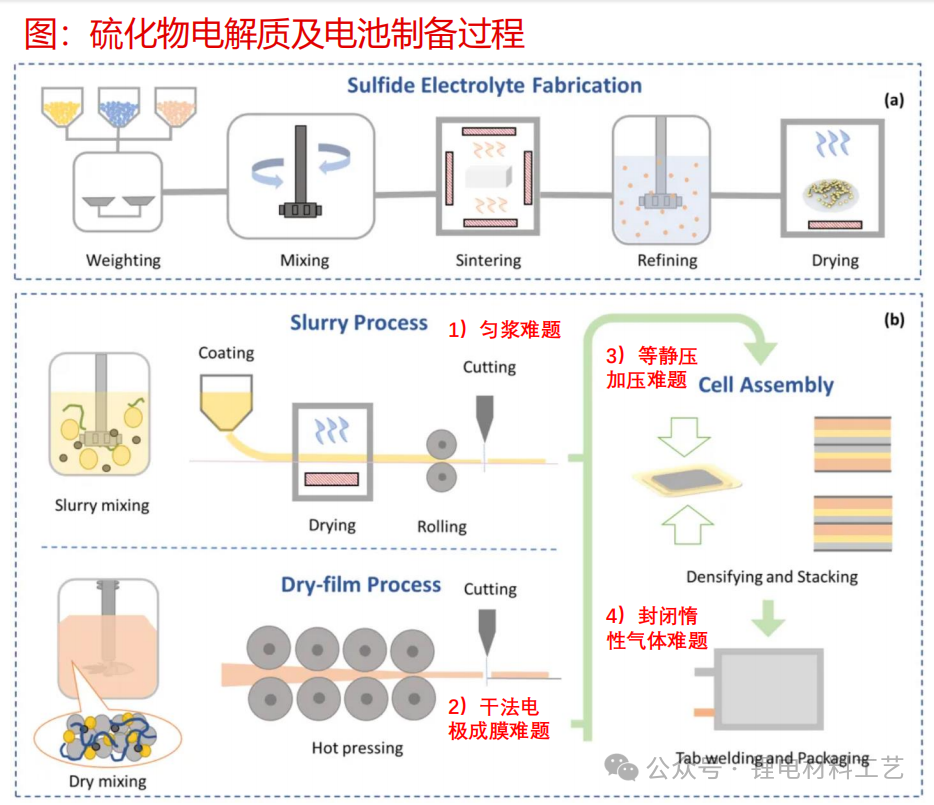
➢ 1) Large-scale production and cost reduction of key solid electrolyte materials: including large-scale planned mass production (g grade to ton), nano-level preparation (um grade to nm grade), continuous production and cost control;
➢ 2) Mass production problems at the electrode sheet level: including mixing (positive and negative electrode materials + solid electrolyte mixture), homogenizing (homogenization problems of wet positive and negative electrodes), dry electrode production
➢ 3) Mass production problems at the battery level: battery production, assembly, and air humidity control
➢ 4) Terminal applications: energy density improvement, battery capacity maintenance, battery cycle life
➢ Sulfide solid electrolytes are currently facing the problem of large-scale production: the consistency of raw materials and particle size control issues. At the same time, sulfide SE is sensitive to air/water, and the sealing of transportation connections is also crucial;
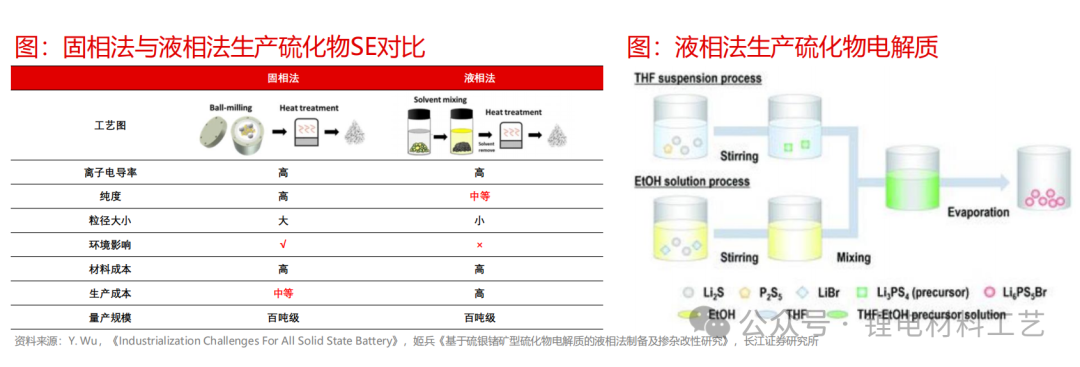
➢ Generally speaking, the preparation methods of sulfide electrolytes can be divided into three categories: 1) Solid phase reaction method: Add raw materials to mix according to the stoichiometric ratio to form the precursor material. The precursor material is heated to melt to the melting point, and then the sample is cooled to room temperature to obtain the electrolyte material. During the reaction conditions, the lithium sulfur volatilizes directly at high temperatures, which is easy to cause miscellaneous matters; 2) Mechanical ball milling method: During the high-speed ball milling process, the raw material particles achieve collision diffusion and reaction during impact; 3) Liquid phase method: Use organic solvents as medium to synthesize sulfide electrolytes. The synthesized electrolyte particles are small and have good consistency, but the solvent is generally a toxic solvent. At the same time, the conductivity of the sulfide electrolyte produced by the liquid phase method is slightly lower than that of the solid phase method.
➢ The raw materials of sulfide electrolyte are generally lithium sulfide, phosphorus pentasulfide and lithium chloride, among which lithium sulfide is the main raw material.
➢ Lithium sulfide is not stable in nature. It is easy to absorb water vapor in the air and hydrolyzes, which releases highly toxic hydrogen sulfide gas. It can be decomposed by acid to release hydrogen sulfide, which can react violently with nitric acid; the difference between battery-grade lithium sulfide and industrial-grade is the different requirements for the purity and particle size of the product.
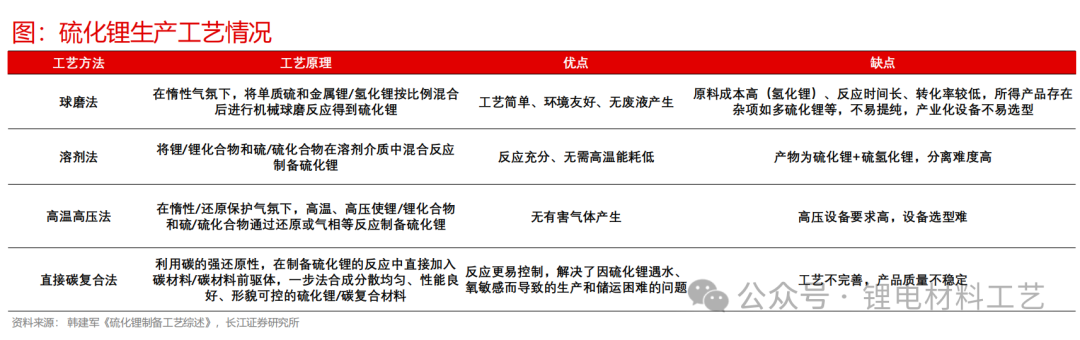
➢ Due to the high price of lithium sulfide and the difficulty in solving the air stability problem, better air protection is required during preparation, the difficulty of sulfide electrolytic production lies in how to achieve large-scale low-cost mass production of lithium sulfide.
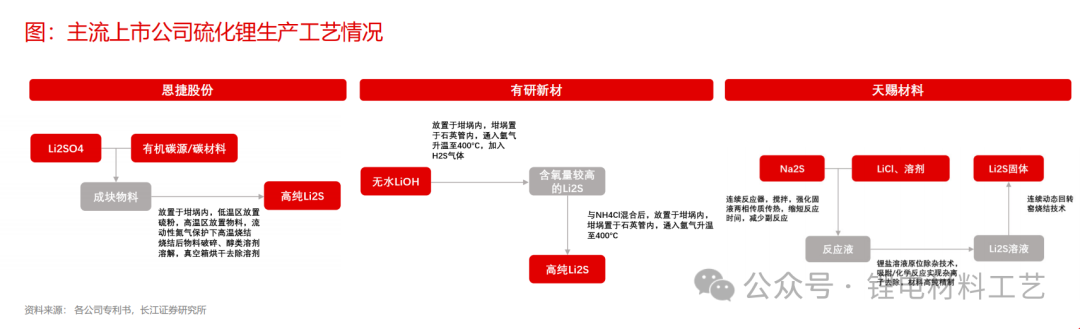
➢ The process routes for mainstream listed companies to layout lithium sulfide have their own advantages, mainly focusing on solid phase methods, liquid phase methods and carbon thermal reduction methods. Among them, the solid phase processes are used to prepare lithium sulfide by the solid phase process, Xiamen Tungsten, Tianci, etc. use liquid phase processes to prepare lithium sulfide and sulfide electrolytes. Enjie Co., Ltd. and Rongbai use carbon thermal reduction methods to prepare lithium sulfide, and use solid phase processes to prepare sulfide electrolytes.
➢ From a cost perspective, there are certain cost advantages in the preparation of lithium sulfide by liquid phase method, but it is still in the early stage of mass production, and the subsequent cost reduction needs further verification.
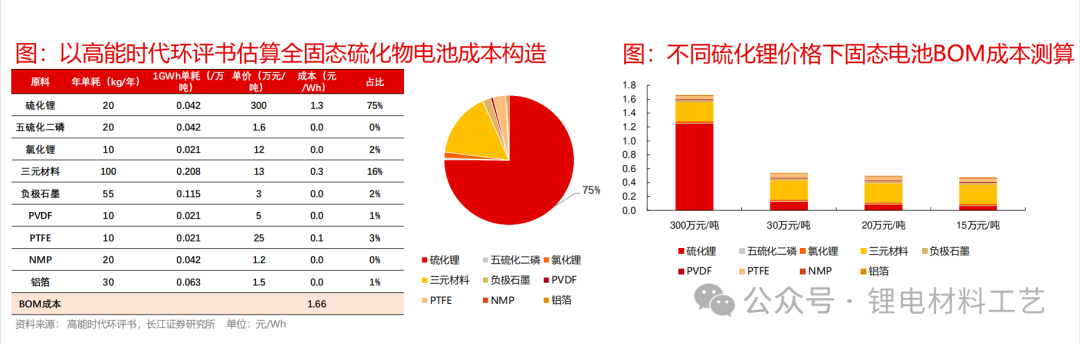
➢ According to the high-energy era 5Ah all-solid-state batteries, the current BOM production cost of 1Wh all-solid-state batteries is 1.66 yuan/Wh. Assuming that the single ton of lithium sulfide is priced at 3 million yuan/ton, the corresponding lithium sulfide production cost accounts for about 75% of the battery production cost. In the future, with the gradual breakthrough of domestic lithium sulfide technology, it is assumed that the price of lithium sulfide drops to 300,000 yuan/ton, and the BOM cost of the corresponding sulfide solid-state batteries will drop to 0.54 yuan/Wh, and there is a lot of room for cost reduction, and it is expected to achieve parity with lithium batteries in the future.
➢ The production of battery composite electrodes and electrode membranes: After the production of sulfide solid electrolytes, the battery electrode sheet is selected for composite electrode sheets, which are composed of active materials, conductive additives and SE. Interface problems will occur in the middle, resulting in an increase in the internal resistance of the electrode and a decrease in capacity. How to overcome the internal resistance and capacity problems is the focus of subsequent improvements. After the materials are mixed in the preparation of composite electrode sheets, how to closely adhere to the current collector is also the problem.
➢ Massive production of sulfide batteries: the difficulty lies in dry electrodes, densification and pressurization, and specific environmental assembly. The difficulty of dry electrodes lies in the fiberization of the adhesive. At present, suitable fibrosis equipment (such as high-speed airflow purge, screw extruder, rolling mill) is still under development; at the same time, the electrode needs tight solid-solid contact, and isostatic pressure of 300MPA is the subsequent problem; finally, sulfide SE is sensitive to air/water, SE material manufacturing may require a closed circulating inert atmosphere, and stricter drying conditions are required during battery manufacturing.
➢ In the industrial chain, lithium sulfide and solid electrolyte production of sulfide and sulfide are all laid out in the production of lithium sulfide from upstream lithium sulfide, midstream material plants and downstream battery plants. In the future, with the breakthrough of domestic lithium sulfide production barriers, the price of lithium sulfide is expected to decline, driving the acceleration of the industrialization of sulfide solid batteries.