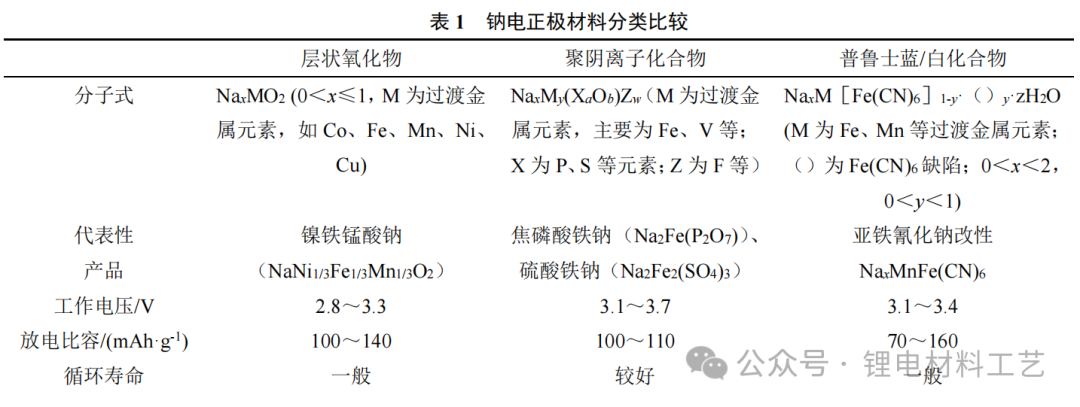
Sodium-electrode materials are divided into three categories, each with its characteristics and face challenges. Different microstructures lead to differences in diffusion paths and energy barriers of sodium ions in the material, thereby affecting electrochemical properties, see Figure 1.
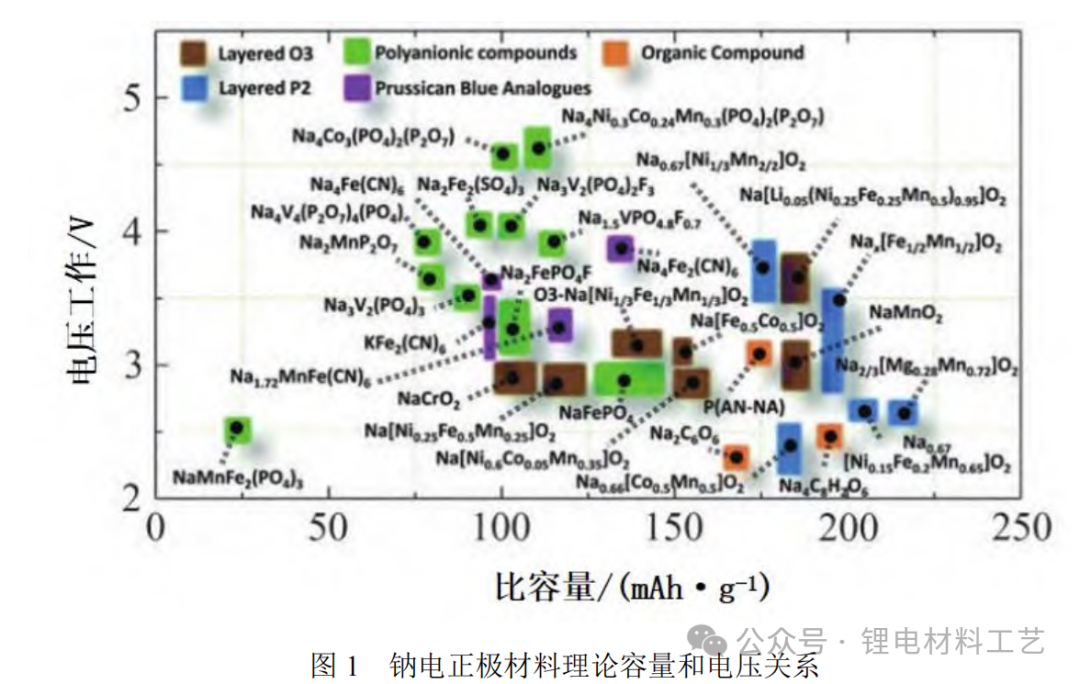
1. P2 and O3-type layered oxides in the positive electrode materials of layered oxide sodium ion batteries have become the focus of research due to their significant development potential. The P2 type positive electrode consists of an ABBA type oxide layer. As shown in Figure 2, sodium ions occupy the triangular prism gap in the sodium layer and migrate through short paths, giving the material high magnification performance. Due to the low initial sodium content, the voltage needs to be increased to 1.5~4.5 V to increase the energy density, but it may accelerate battery attenuation. Limiting voltages between 2.0 and 4.0 V can improve cycle stability, but will sacrifice some capacity. Therefore, the characteristics of these materials need to be comprehensively considered in battery design and application selection to achieve optimal performance.
The O3 type sodium electropositive electrode material structure is ABCABC type layered. As shown in Figure 2, sodium ions are mainly embedded in the octahedral position of the sodium layer. Since sodium ions need to pass a longer migration path, the rate performance of O3-type materials is usually not as good as that of P2-type. O3 type materials have high initial sodium content and high energy density, and can maintain excellent cycling stability at low voltages of 2.0 to 4.0 V. These materials are simple to synthesis and environmentally friendly, and are compatible with the existing lithium-ion battery ternary material production lines, which is conducive to large-scale production. Therefore, O3-type layered transition metal oxides have broad application prospects in the commercialization of sodium ion batteries.
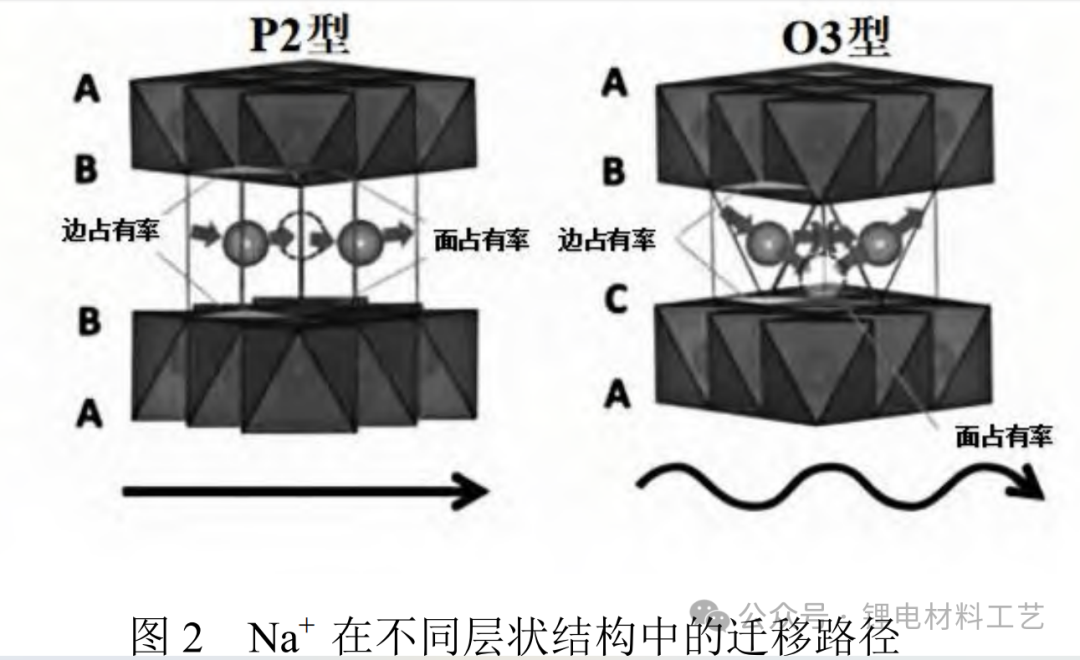
1.2 Polyanionic compounds This type of substance is composed of anionic unit (XO4)n- composed of elements such as boron, phosphorus, sulfur, silicon, and other elements and its derivatives (XmO3m+1)n-. These materials increase the working voltage through inducing effects, which in turn affects chemical and electrochemical properties. Its robust 3D frame structure helps reduce structural changes when sodium ions are deintercalated, thereby improving the structural stability of the material. In addition, strong X-O covalent bonds inhibit oxygen evolution reactions, thereby improving material circulation stability and safety [10]. Vanadium-based polyanionic materials provide operating voltages comparable to lithium-ion batteries under certain conditions. NASICON structural electrodes are favored for their stability and ionic conductivity, but their low conductivity and limited capacity limit their application. Figure 3 shows the structural characteristics of NASICON type polyanionic compounds.
1.3 Prussian Blue/White Compound Prussian Blue/White is a rookie in sodium electropositive electrode materials and has attracted much attention. These compounds have a face-centered cubic structure, in which transition metal ions form hexagonal coordination with CN ligands, and sodium ions are distributed in three-dimensional channels and pores [13]. The large gap space of its open frame structure not only gives high theoretical capacity, but also has advantages such as non-toxicity. Figure 4 shows the crystal structures of 3 Prussian blue analogues.
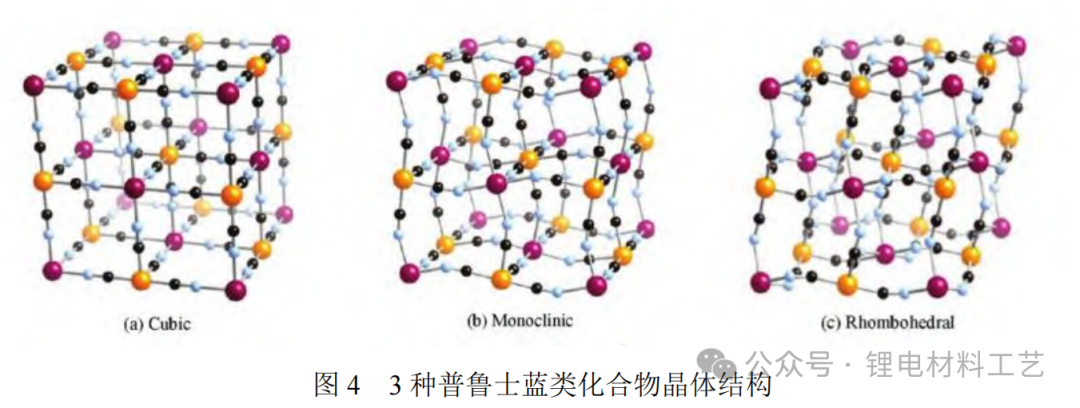
In addition, Prussian blue/white compounds have the lowest cost, high energy density, and open three-dimensional structures are conducive to sodium ion deintercalation, safety and rate-efficiency. These factors further promote their widespread application. However, such materials also face some challenges in practical applications. Due to the internal structure, these materials have low tap density and difficult to remove crystallization water, resulting in poor thermal cycle stability and prone to thermal defects, affecting thermal capacity and electrochemical properties. High thermal energy during charging and discharging may accelerate the dissolution of transition metal ions, causing safety problems. For details of the classification of sodium electropositive electrode materials, see Table 1.